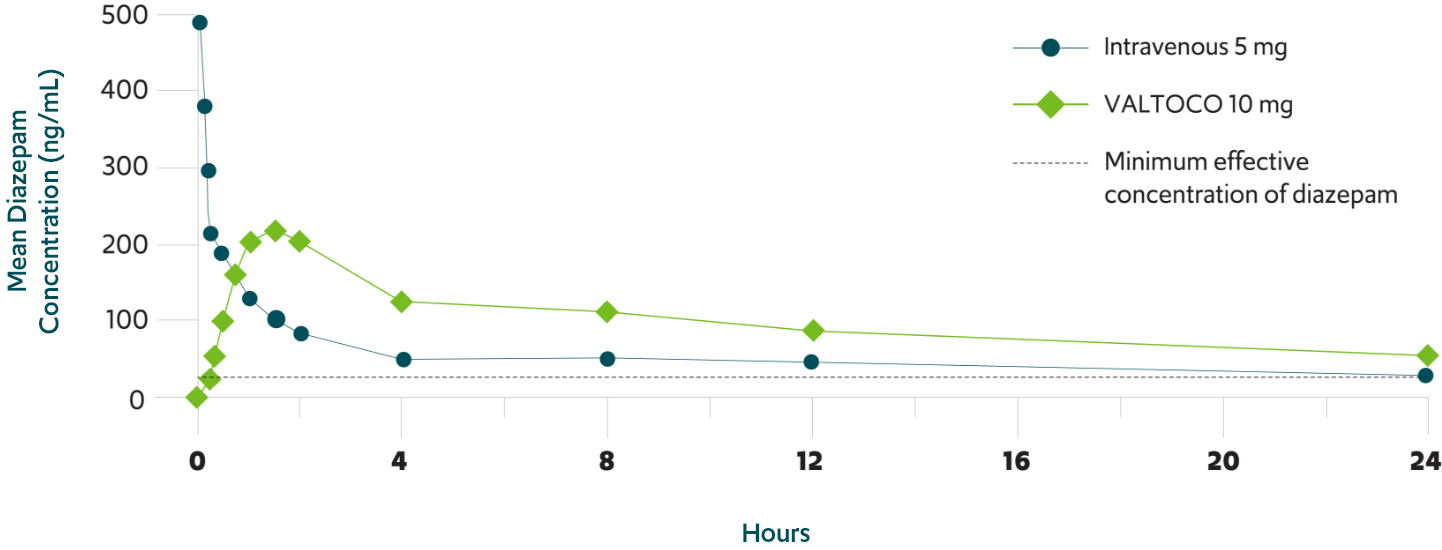
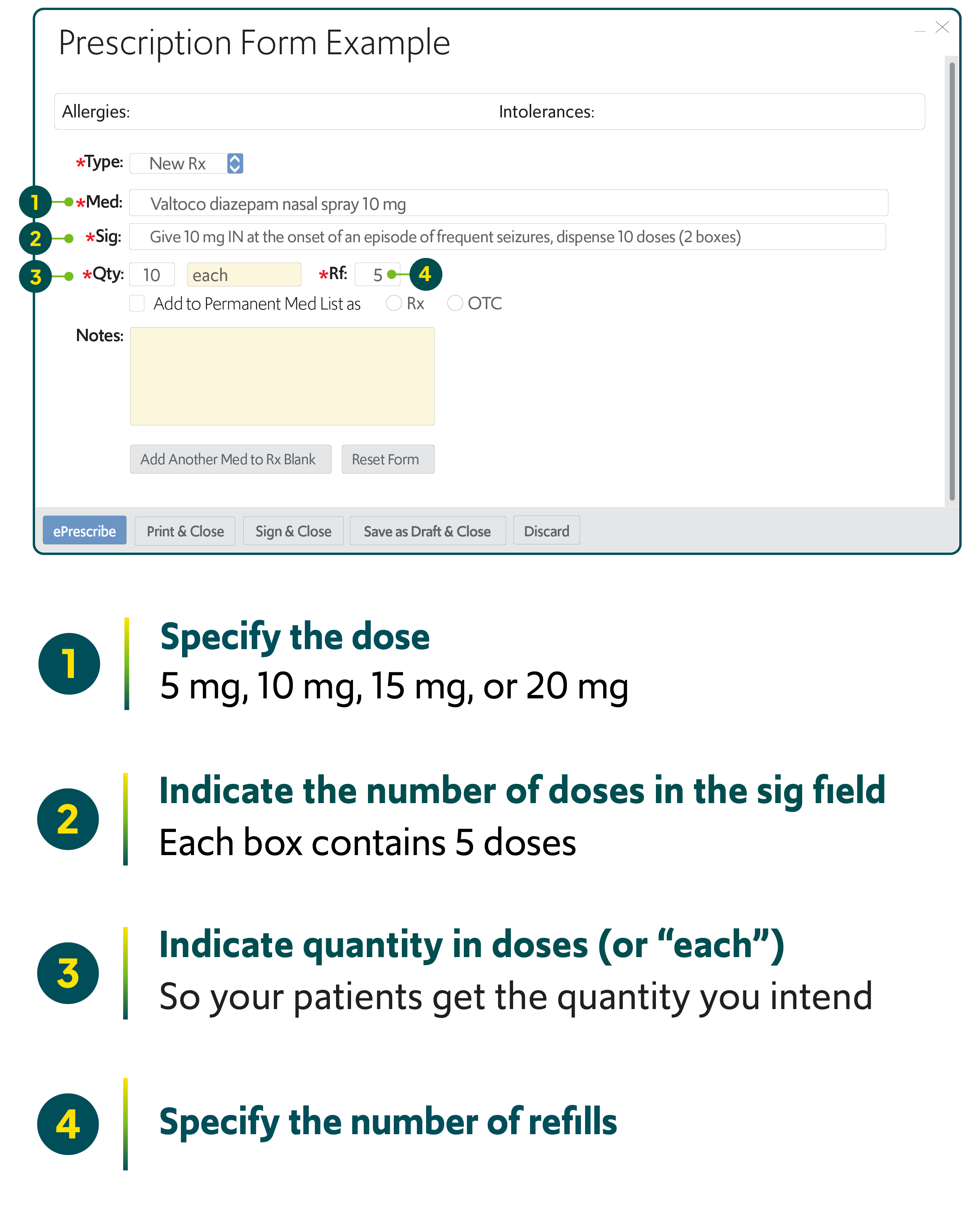
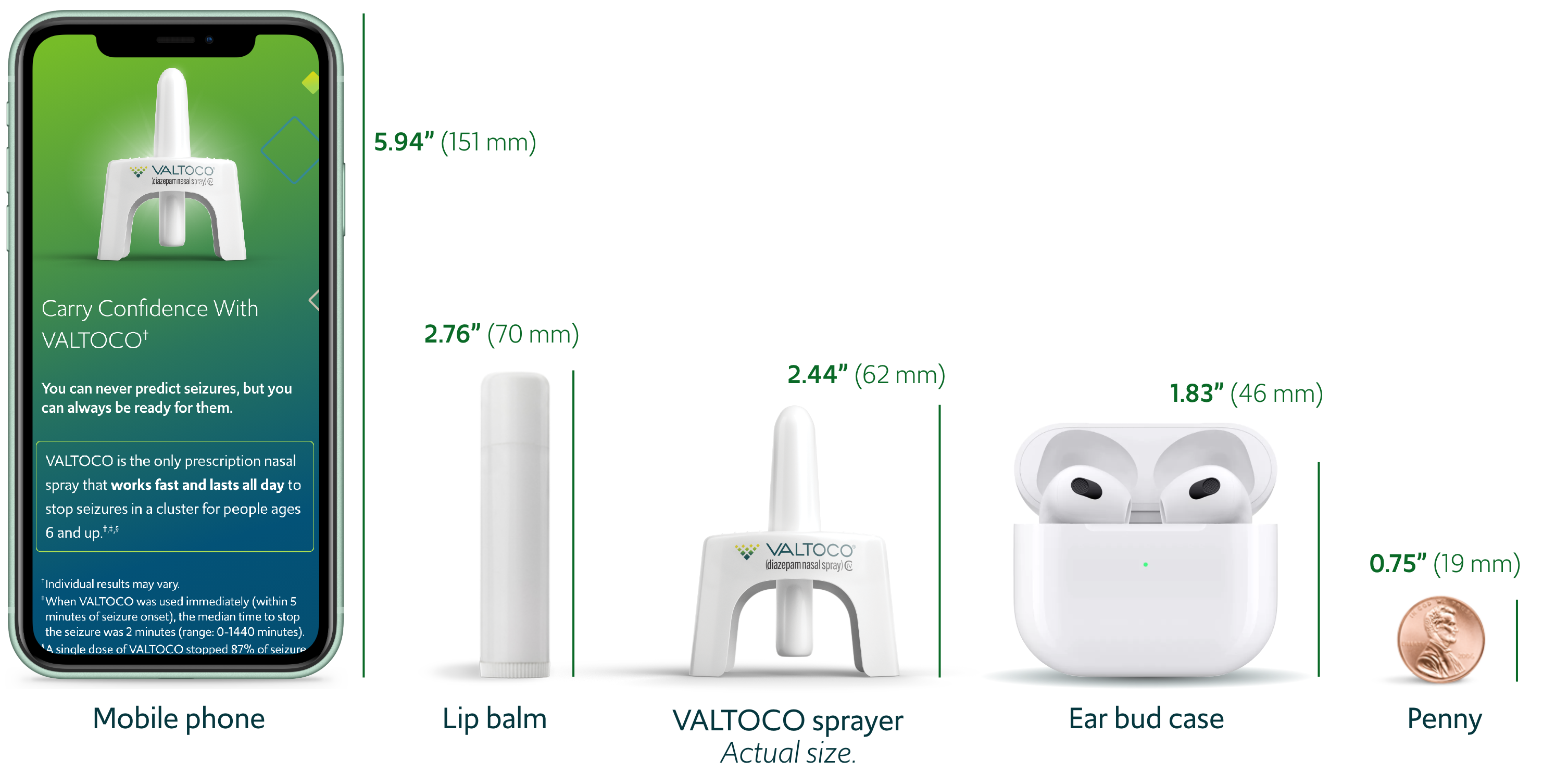
Valtoco Dose Chart
Valtoco Dose Chart - Notifique los efectos secundarios de los fármacos recetados a la administración de. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Valtoco® (diazepam nasal spray) is not approved for use in neonates or infants. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,. Valtoco® (diazepam nasal spray) is not approved for use in neonates or infants. Notifique los efectos secundarios de los fármacos recetados a la administración de. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1.. Notifique los efectos secundarios de los fármacos recetados a la administración de. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older. Use of valtoco, particularly in patients at elevated risk,. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. Valtoco® (diazepam nasal spray) is indicated. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. Valtoco® (diazepam nasal spray) is not approved for use in neonates or infants. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Valtoco is the only prescription nasal spray. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,. Notifique los efectos secundarios de los fármacos recetados a la administración de. Valtoco® (diazepam nasal spray) is not approved for use in neonates or infants. Report side effects of prescription drugs. Valtoco® (diazepam nasal spray) is not approved for use in neonates or infants. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older.. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,. Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older. Report side effects of prescription drugs to the. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,. Notifique los efectos secundarios de los fármacos. Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Notifique los efectos secundarios de. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Valtoco is the only prescription nasal spray used to immediately treat episodes of frequent seizures in adults and children 2 years and older. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. Valtoco® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures). Report side effects of prescription drugs to the fda by visiting www.fda.gov/medwatch or by calling 1. Valtoco® (diazepam nasal spray) is not approved for use in neonates or infants. Use of valtoco, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of valtoco along with monitoring for signs and symptoms of abuse, misuse,.Diazepam Nasal Spray (Valtoco) LGS Foundation
Support for People Prescribed VALTOCO® (diazepam nasal spray)
Why choose VALTOCO® diazepam nasal spray CIV?
New Drug Valtoco Approved as Rescue Therapy for Seizures
VALTOCO® diazepam nasal spray CIV dosing individualized for adult and pediatric use
Support for People Prescribed VALTOCO® (diazepam nasal spray)
What you need to know about VALTOCO® (diazepam nasal spray) CIV
Valtoco Nasal Spray Now Available for Seizure Clusters MPR
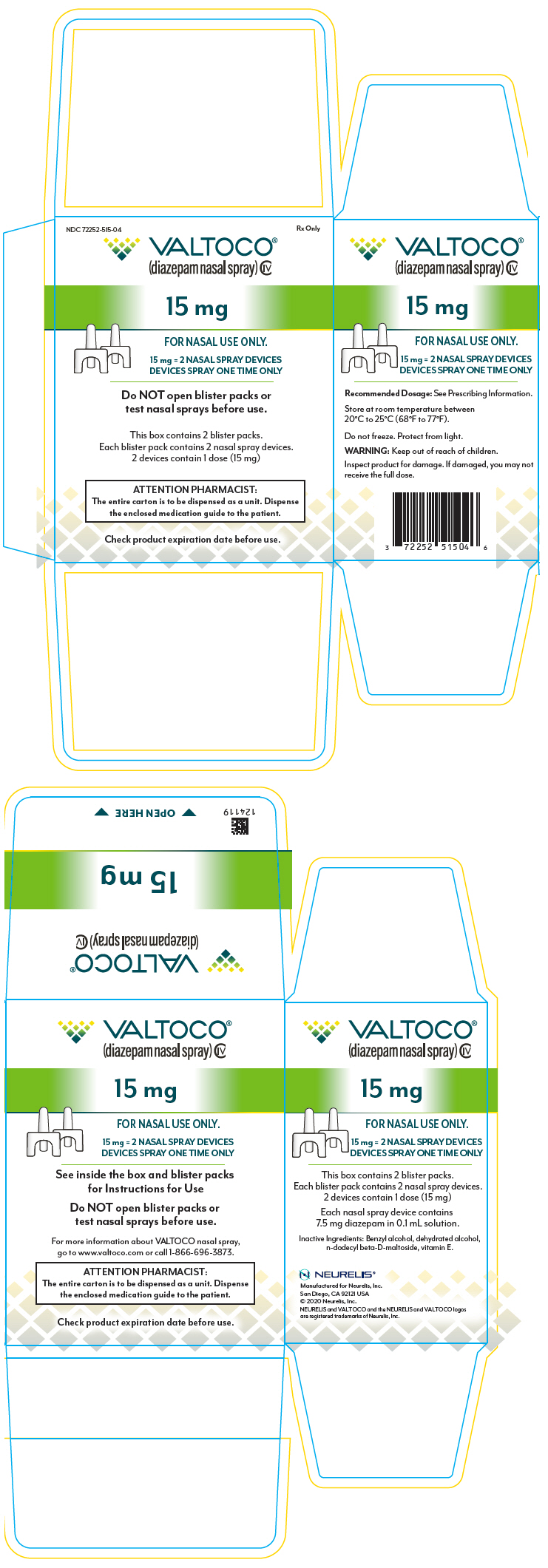
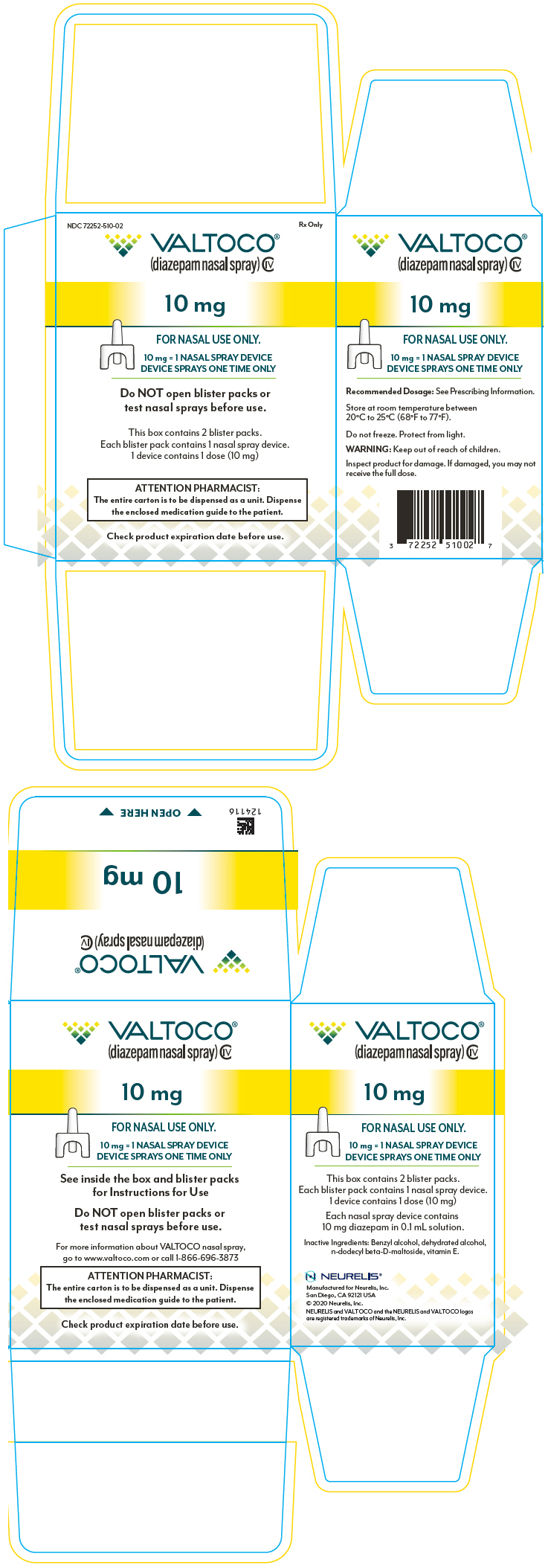
DailyMed VALTOCO diazepam spray
DailyMed VALTOCO diazepam spray
Notifique Los Efectos Secundarios De Los Fármacos Recetados A La Administración De.
Valtoco Is The Only Prescription Nasal Spray Used To Immediately Treat Episodes Of Frequent Seizures In Adults And Children 2 Years And Older.
Related Post: