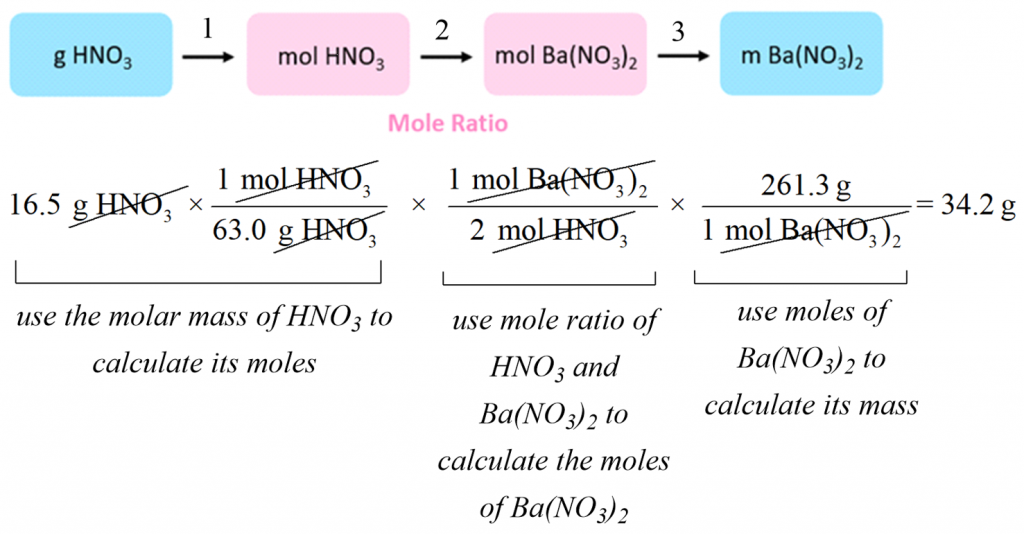
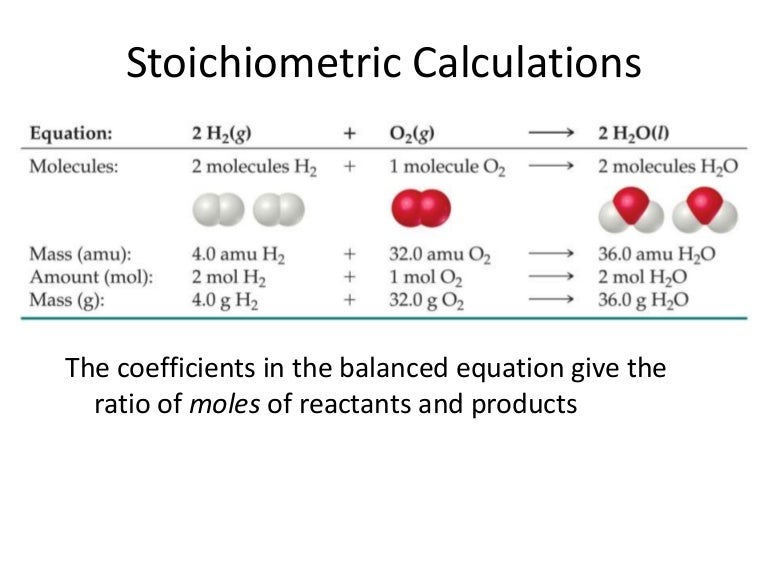
Stoichiometric Chart
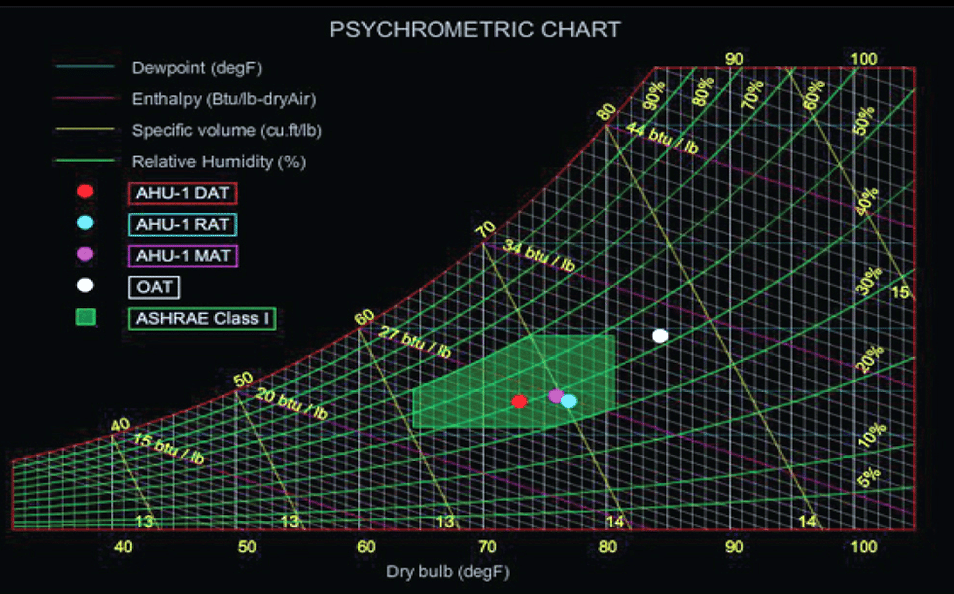
Stoichiometric Chart - Learn the stoichiometry definition and the connection between chemistry and stoichiometry. German chemist jeremias benjamin richter was the first to define. Stoichiometry is a fundamental concept in chemistry that allows us to calculate the amounts of reactants and products using balanced chemical equations. Stoichiometry in chemistry is the process of calculating of masses (sometimes volumes also) of reactants and products involved in a chemical reaction. Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction). Explore stoichiometry formulas, mole ratios, and stoichiometry examples. Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions. Stoichiometry (/ ˌstɔɪkiˈɒmɪtri / ⓘ) is the relationships between the masses of reactants and products before, during, and following chemical reactions. Stoichiometry is the study of the relationship between the quantity of reactants and products in a chemical reaction. When chemists conduct experiments, they need. When chemists conduct experiments, they need. The meaning of stoichiometric is of, relating to, used in, or marked by stoichiometry. Stoichiometry (/ ˌstɔɪkiˈɒmɪtri / ⓘ) is the relationships between the masses of reactants and products before, during, and following chemical reactions. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. Stoichiometry is a fundamental concept in chemistry that allows us to calculate the amounts of reactants and products using balanced chemical equations. Stoichiometry is the study of the relationship between the quantity of reactants and products in a chemical reaction. Learn the stoichiometry definition and the connection between chemistry and stoichiometry. Stoichiometry in chemistry is the process of calculating of masses (sometimes volumes also) of reactants and products involved in a chemical reaction. Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction). It involves calculations that take into account the masses of reactants and products in a given chemical. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. It involves calculations that take into account the masses of reactants and products in a given chemical. German chemist jeremias benjamin richter was the first to define. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. Stoichiometry is the study of the. Stoichiometry is a fundamental concept in chemistry that allows us to calculate the amounts of reactants and products using balanced chemical equations. Stoichiometry (/ ˌstɔɪkiˈɒmɪtri / ⓘ) is the relationships between the masses of reactants and products before, during, and following chemical reactions. It involves calculations that take into account the masses of reactants and products in a given chemical.. Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions. It involves calculations that take into account the masses of reactants and products in a given chemical. The meaning of stoichiometric is of, relating to, used in, or marked by stoichiometry. German chemist jeremias benjamin richter was the first to define. Explore stoichiometry formulas, mole ratios,. Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. When chemists conduct experiments, they need. Stoichiometry in chemistry is the process of calculating of masses (sometimes volumes also) of reactants and products involved in a chemical. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. Stoichiometry is the study of the relationship between the quantity of reactants and products in a chemical reaction. Learn the stoichiometry definition and the connection between chemistry and stoichiometry. Stoichiometry is the measure of the elements within a reaction. When chemists conduct experiments, they need. It involves calculations that take into account the masses of reactants and products in a given chemical. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction).. Stoichiometry (/ ˌstɔɪkiˈɒmɪtri / ⓘ) is the relationships between the masses of reactants and products before, during, and following chemical reactions. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. German chemist jeremias benjamin richter was the first to define. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. When chemists. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. German chemist jeremias benjamin richter was the first to define. Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction). Stoichiometry is a fundamental concept in chemistry that allows us to calculate the amounts of reactants and products. Stoichiometry is the measure of the elements within a reaction. The meaning of stoichiometric is of, relating to, used in, or marked by stoichiometry. German chemist jeremias benjamin richter was the first to define. It involves calculations that take into account the masses of reactants and products in a given chemical. Stoichiometry is the study of the relationship between the. German chemist jeremias benjamin richter was the first to define. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. Stoichiometry in chemistry is the process of calculating of masses (sometimes volumes also) of reactants and products involved in a chemical reaction. Stoichiometry (/ ˌstɔɪkiˈɒmɪtri / ⓘ) is the relationships between the masses of reactants and products before, during, and following chemical. Explore stoichiometry formulas, mole ratios, and stoichiometry examples. When chemists conduct experiments, they need. Stoichiometry is a fundamental concept in chemistry that allows us to calculate the amounts of reactants and products using balanced chemical equations. Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions. The meaning of stoichiometric is of, relating to, used in, or marked by stoichiometry. It involves calculations that take into account the masses of reactants and products in a given chemical. Stoichiometry is the study of the relationship between the quantity of reactants and products in a chemical reaction. Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction). German chemist jeremias benjamin richter was the first to define. Stoichiometry in chemistry is the process of calculating of masses (sometimes volumes also) of reactants and products involved in a chemical reaction. Stoichiometry (/ ˌstɔɪkiˈɒmɪtri / ⓘ) is the relationships between the masses of reactants and products before, during, and following chemical reactions.How to Read a Psychrometric Chart Nlyte
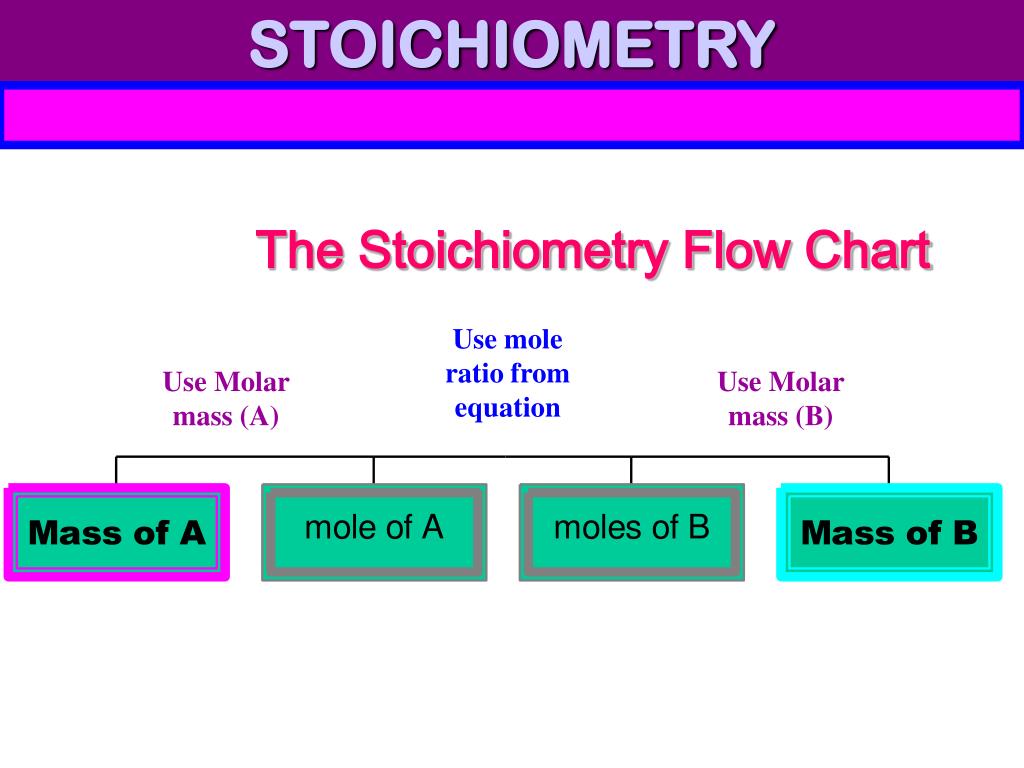
PPT STOICHIOMETRY PowerPoint Presentation, free download ID4499255
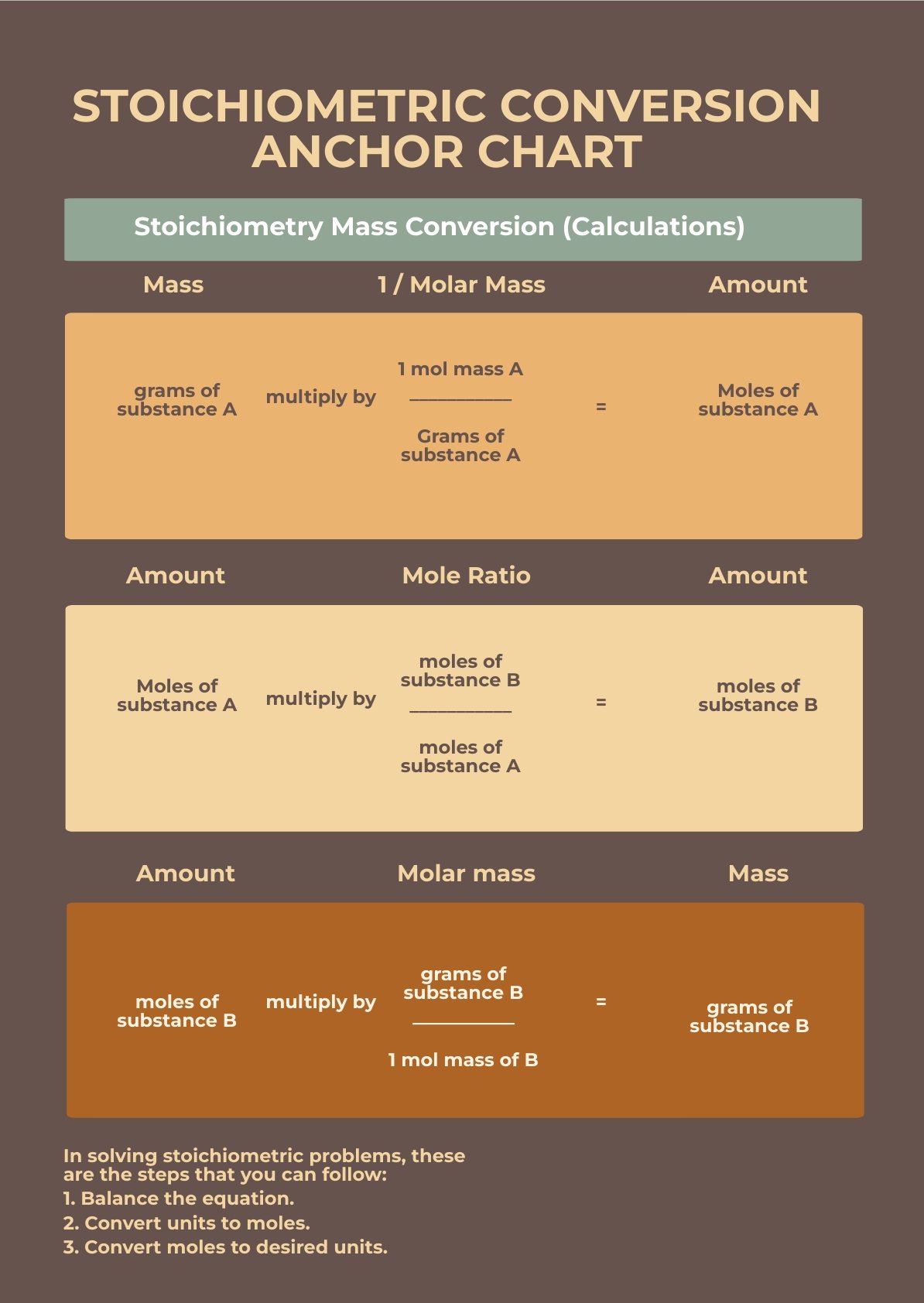
Stoichiometric Conversion Anchor Chart in PDF, Illustrator Download
What is Stoichiometry? Balancing Equations
The Stoichiometric Chart YouTube
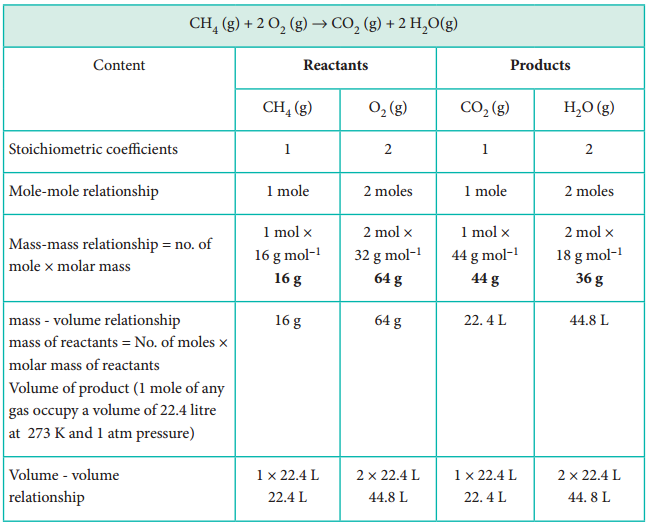
Stoichiometry of Chemical Reactions Chemistry Steps
What Is A Stoichiometry Calculator And How Should You vrogue.co
Extended Reaction Stoichiometry Road Map — Examples Expii
Gasoline Stoichiometric Ratio
Stoich Conversion Chart
Learn The Stoichiometry Definition And The Connection Between Chemistry And Stoichiometry.
At Its Core, Stoichiometry Is The Study Of The Quantitative Relationships Between The Reactants And Products In Chemical Reactions.
Stoichiometry Is The Measure Of The Elements Within A Reaction.
Related Post: