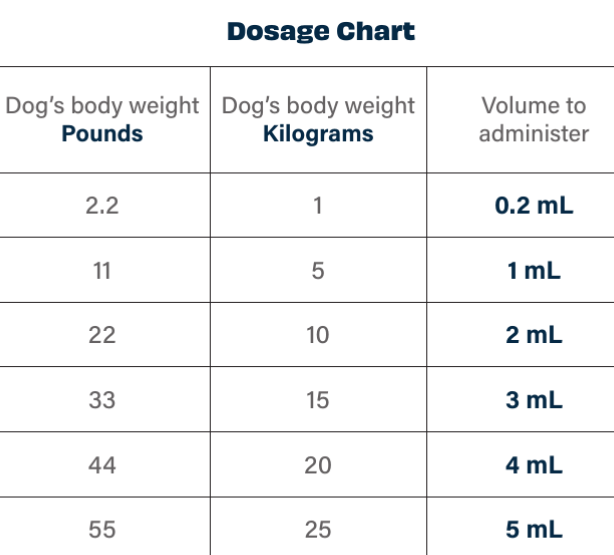
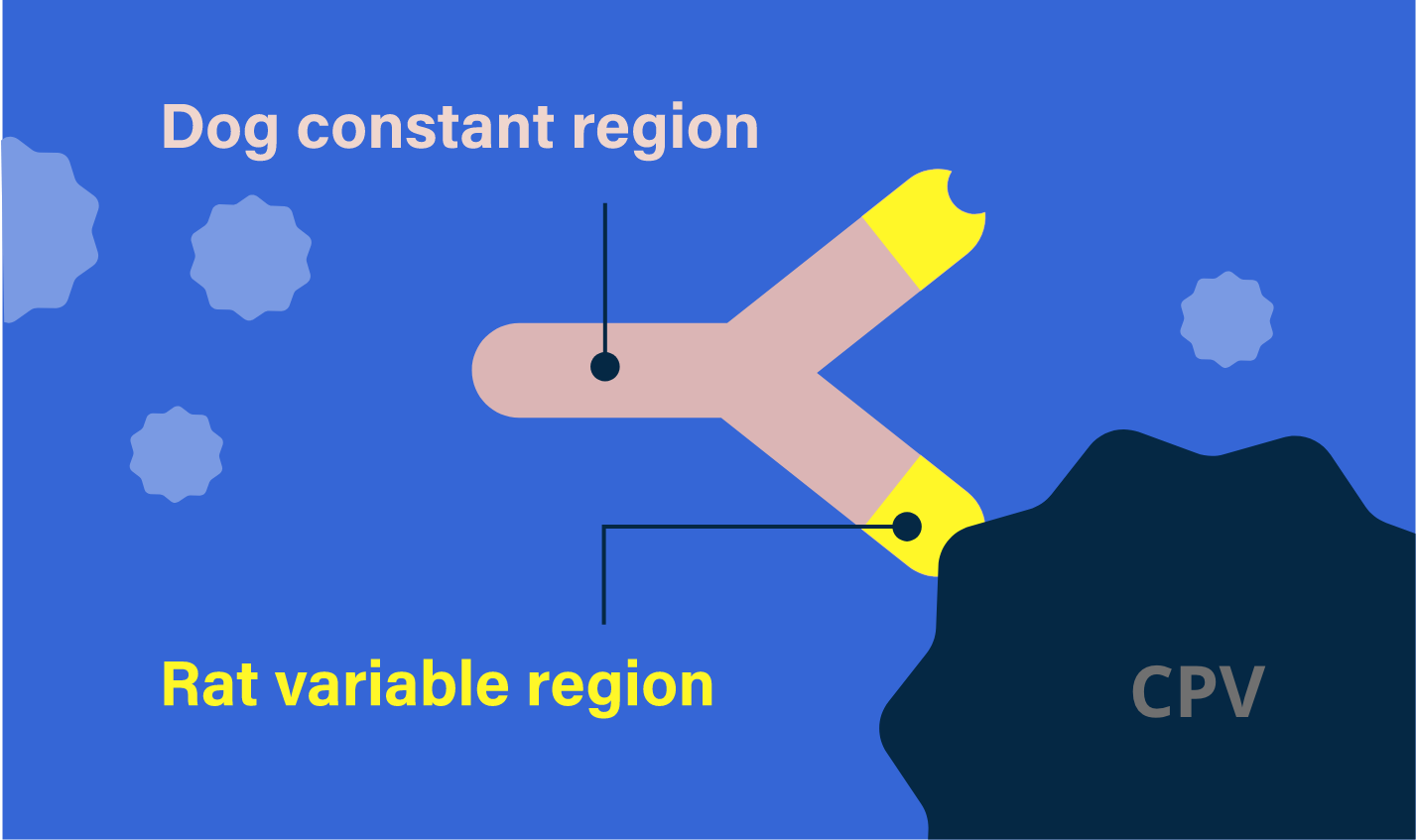
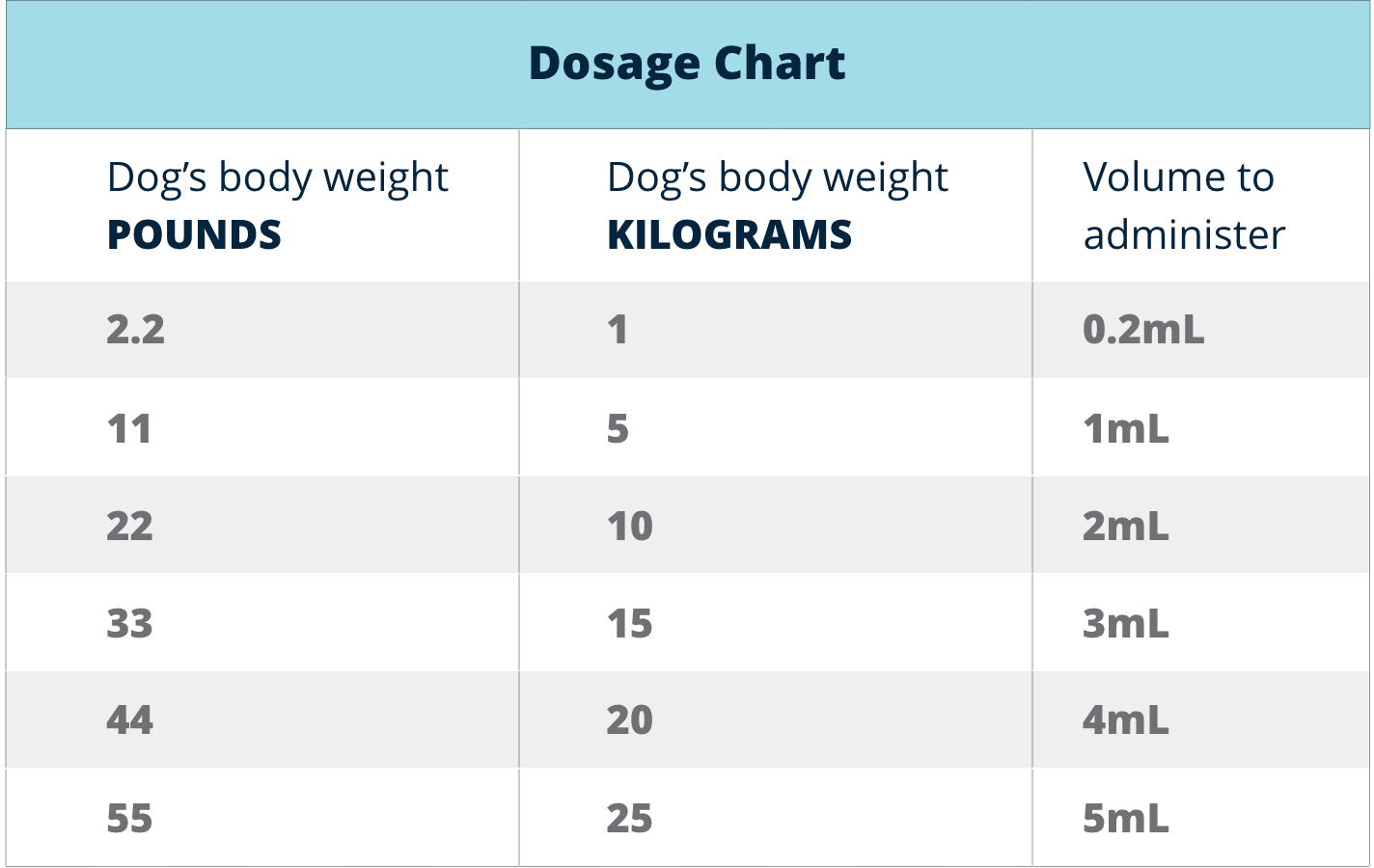
Parvo Monoclonal Antibody Dosing Chart
Parvo Monoclonal Antibody Dosing Chart - In 2023, a targeted canine parvovirus monoclonal antibody (cpma) became commercially available through usda conditional approval. Canine parvovirus monoclonal antibody (cpma) cpma is conditionally approved by the usda for treatment of canine parvovirus in dogs 8 weeks of age and older. The product, canine parvovirus monoclonal antibody, selectively binds and neutralizes canine parvovirus. Examples of the volume to administer are. Canine parvovirus monoclonal antibody is composed of a dog constant region and a rat variable region. These two elements work together to neutralize canine parvovirus in vivo by selectively. Elanco’s canine parvovirus monoclonal antibody is the first therapeutic solution to receive conditional usda approval for the treatment of canine parvovirus. This product is a sterile liquid containing a chimeric monoclonal antibody (rat. The cpma is a chimeric antibody, meaning it. Targets parvovirus directly helps decrease burden of. The cpma is a chimeric antibody, meaning it. With just one intravenous dose, canine parvovirus monoclonal antibody may shorten the course of the disease and improve outcome. In 2023, a targeted canine parvovirus monoclonal antibody (cpma) became commercially available through usda conditional approval. Canine parvovirus monoclonal antibody (cpma) cpma is conditionally approved by the usda for treatment of canine parvovirus in dogs 8 weeks of age and older. Elanco’s canine parvovirus monoclonal antibody is the first therapeutic solution to receive conditional usda approval for the treatment of canine parvovirus. These two elements work together to neutralize canine parvovirus in vivo by selectively. Canine parvovirus monoclonal antibody is composed of a dog constant region and a rat variable region. The product, canine parvovirus monoclonal antibody, selectively binds and neutralizes canine parvovirus. And allow dogs to return. Learn about management, treatment methods, and its potential to improve outcomes, team morale,. And allow dogs to return. Examples of the volume to administer are. Targets parvovirus directly helps decrease burden of. Canine parvovirus monoclonal antibody is composed of a dog constant region and a rat variable region. Elanco’s canine parvovirus monoclonal antibody is the first therapeutic solution to receive conditional usda approval for the treatment of canine parvovirus. And allow dogs to return. Explore the impact of canine parvovirus monoclonal antibody (cpma) on parvo cases. In 2023, a targeted canine parvovirus monoclonal antibody (cpma) became commercially available through usda conditional approval. Canine parvovirus monoclonal antibody (cpma) cpma is conditionally approved by the usda for treatment of canine parvovirus in dogs 8 weeks of age and older. Learn about. This product is a sterile liquid containing a chimeric monoclonal antibody (rat. The product, canine parvovirus monoclonal antibody, selectively binds and neutralizes canine parvovirus. Explore the impact of canine parvovirus monoclonal antibody (cpma) on parvo cases. The cpma is a chimeric antibody, meaning it. Targets parvovirus directly helps decrease burden of. Examples of the volume to administer are. Elanco’s canine parvovirus monoclonal antibody is the first therapeutic solution to receive conditional usda approval for the treatment of canine parvovirus. Explore the impact of canine parvovirus monoclonal antibody (cpma) on parvo cases. The cpma is a chimeric antibody, meaning it. This product is a sterile liquid containing a chimeric monoclonal antibody (rat. Examples of the volume to administer are. Targets parvovirus directly helps decrease burden of. Explore the impact of canine parvovirus monoclonal antibody (cpma) on parvo cases. And allow dogs to return. This product is a sterile liquid containing a chimeric monoclonal antibody (rat. With just one intravenous dose, canine parvovirus monoclonal antibody may shorten the course of the disease and improve outcome. Canine parvovirus monoclonal antibody is composed of a dog constant region and a rat variable region. In 2023, a targeted canine parvovirus monoclonal antibody (cpma) became commercially available through usda conditional approval. Explore the impact of canine parvovirus monoclonal antibody (cpma). These two elements work together to neutralize canine parvovirus in vivo by selectively. Examples of the volume to administer are. And allow dogs to return. Explore the impact of canine parvovirus monoclonal antibody (cpma) on parvo cases. With just one intravenous dose, canine parvovirus monoclonal antibody may shorten the course of the disease and improve outcome. Canine parvovirus monoclonal antibody (cpma) cpma is conditionally approved by the usda for treatment of canine parvovirus in dogs 8 weeks of age and older. This product is a sterile liquid containing a chimeric monoclonal antibody (rat. The product, canine parvovirus monoclonal antibody, selectively binds and neutralizes canine parvovirus. The cpma is a chimeric antibody, meaning it. These two elements. Targets parvovirus directly helps decrease burden of. Canine parvovirus monoclonal antibody (cpma) cpma is conditionally approved by the usda for treatment of canine parvovirus in dogs 8 weeks of age and older. Elanco’s canine parvovirus monoclonal antibody is the first therapeutic solution to receive conditional usda approval for the treatment of canine parvovirus. This product is a sterile liquid containing. The product, canine parvovirus monoclonal antibody, selectively binds and neutralizes canine parvovirus. Learn about management, treatment methods, and its potential to improve outcomes, team morale,. In 2023, a targeted canine parvovirus monoclonal antibody (cpma) became commercially available through usda conditional approval. These two elements work together to neutralize canine parvovirus in vivo by selectively. Targets parvovirus directly helps decrease burden. Targets parvovirus directly helps decrease burden of. Explore the impact of canine parvovirus monoclonal antibody (cpma) on parvo cases. These two elements work together to neutralize canine parvovirus in vivo by selectively. Elanco’s canine parvovirus monoclonal antibody is the first therapeutic solution to receive conditional usda approval for the treatment of canine parvovirus. Canine parvovirus monoclonal antibody is composed of a dog constant region and a rat variable region. With just one intravenous dose, canine parvovirus monoclonal antibody may shorten the course of the disease and improve outcome. And allow dogs to return. The product, canine parvovirus monoclonal antibody, selectively binds and neutralizes canine parvovirus. This product is a sterile liquid containing a chimeric monoclonal antibody (rat. In 2023, a targeted canine parvovirus monoclonal antibody (cpma) became commercially available through usda conditional approval. Examples of the volume to administer are.Early administration of canine parvovirus monoclonal antibody prevented mortality after
Presence of parvovirusspecific IgG class antibodies in patients and... Download Scientific
Early administration of canine parvovirus monoclonal antibody prevented mortality after
Early administration of canine parvovirus monoclonal antibody prevented mortality after
Canine Parvovirus Monoclonal Antibody
Fight Canine Parvovirus
Fight Canine Parvovirus
Canine Parvovirus Monoclonal Antibody
Canine Parvovirus Monoclonal Antibody
Fight Canine Parvovirus
Canine Parvovirus Monoclonal Antibody (Cpma) Cpma Is Conditionally Approved By The Usda For Treatment Of Canine Parvovirus In Dogs 8 Weeks Of Age And Older.
Learn About Management, Treatment Methods, And Its Potential To Improve Outcomes, Team Morale,.
The Cpma Is A Chimeric Antibody, Meaning It.
Related Post: