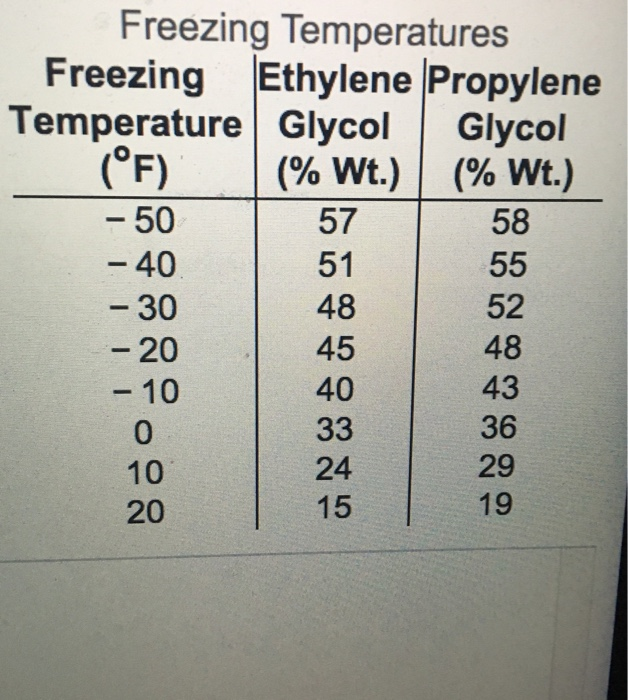
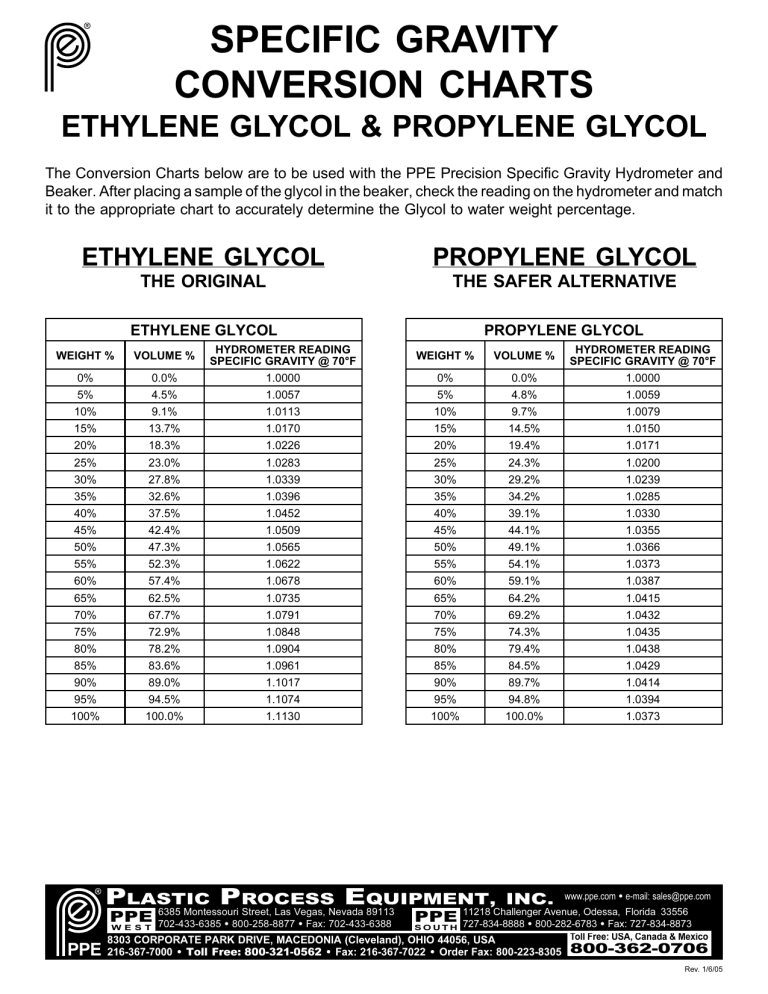
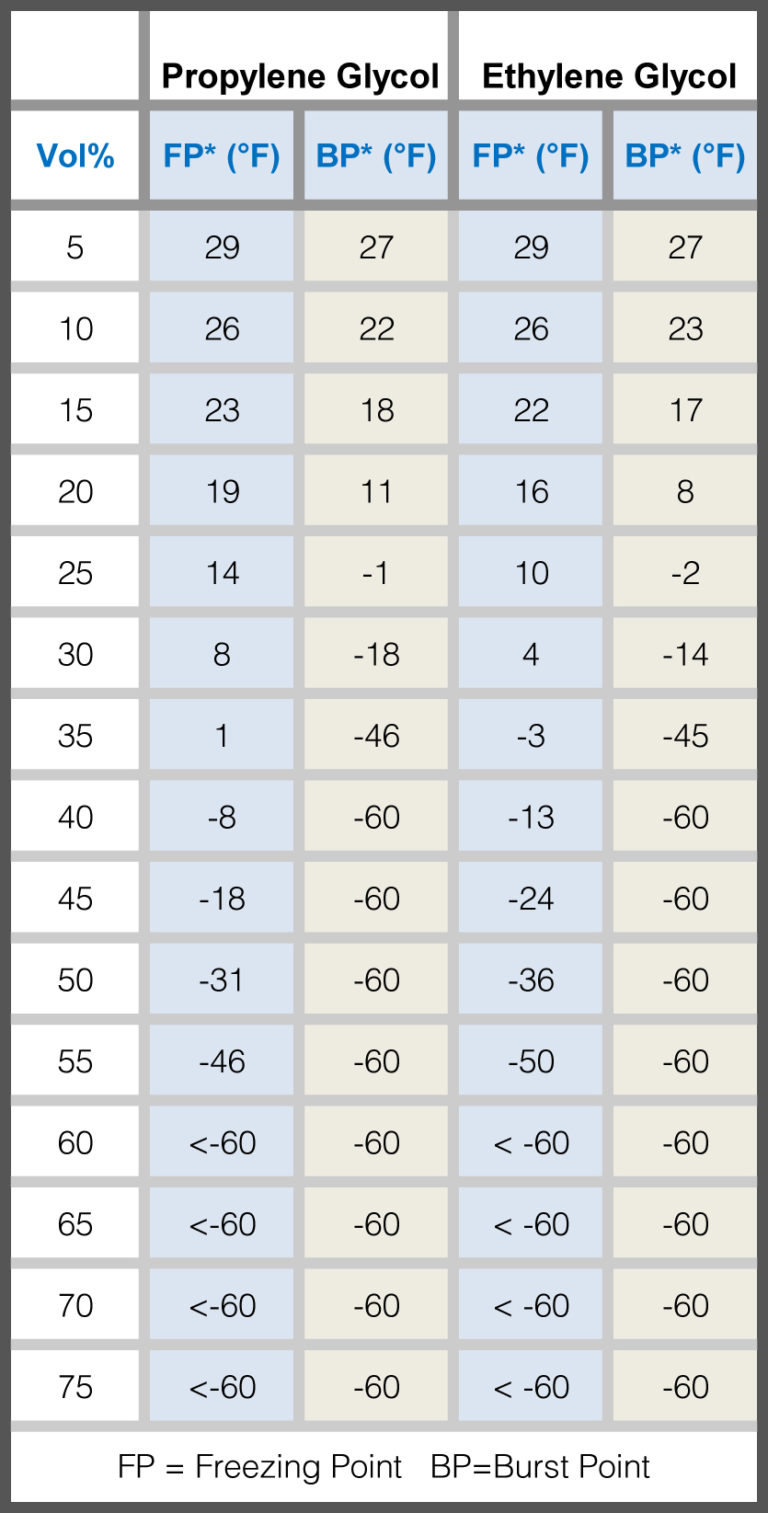
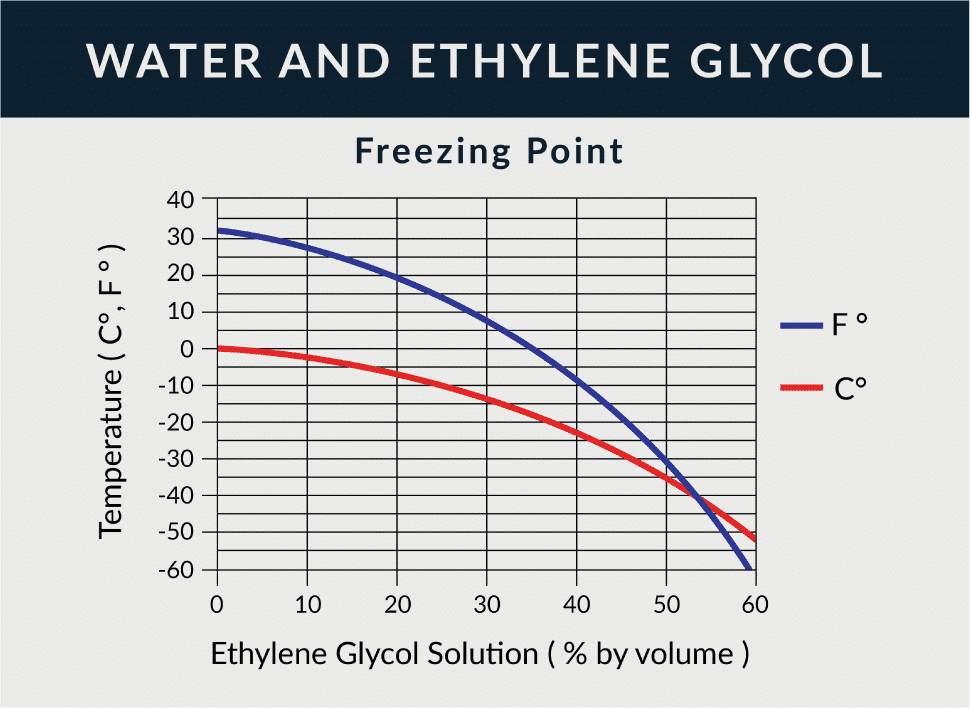
Ethylene Glycol Freezing Point Chart
Ethylene Glycol Freezing Point Chart - Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. Natural sources of ethylene include both natural gas and. Under high pressure, and in the presence of a catalytic metal (platinum, rhodium, nickel), hydrogen reacts with ethylene to form ethane. Ethylene is used primarily as an intermediate in. Ethylene is used in the production of fabricated plastics, antifreeze; This explains why some fruits ripen faster if. Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type of hydrocarbon characterized by at least one carbon. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 Ethylene (ethene), a gas of the formula ch 2 =ch 2, produced by fruit as a hormone to speed ripening of climacteric fruits. 乙烯 (英語: ethylene)是由两个 碳 原子和四个 氢 原子组成的化合物。 两个碳原子之间用双键连接。 Natural sources of ethylene include both natural gas and. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 乙烯 (英語: ethylene)是由两个 碳 原子和四个 氢 原子组成的化合物。 两个碳原子之间用双键连接。 Ethylene (ethene), a gas of the formula ch 2 =ch 2, produced by fruit as a hormone to speed ripening of climacteric fruits. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. Under high pressure, and in the presence of a catalytic metal (platinum, rhodium, nickel), hydrogen reacts with ethylene to form ethane. Ethylene is used in the production of fabricated plastics, antifreeze; Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type of hydrocarbon characterized by at least one carbon. This explains why some fruits ripen faster if. Ethylene is used primarily as an intermediate in. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 乙烯 (英語: ethylene)是由两个 碳 原子和四个 氢 原子组成的化合物。 两个碳原子之间用双键连接。 Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type of hydrocarbon characterized by at least one carbon. This explains why some fruits ripen faster if. Under high pressure, and in the presence of a catalytic metal. Ethylene is used in the production of fabricated plastics, antifreeze; Ethylene (ethene), a gas of the formula ch 2 =ch 2, produced by fruit as a hormone to speed ripening of climacteric fruits. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. 最早报道的植物对乙烯的反应是“. This explains why some fruits ripen faster if. To manufacture ethylene oxide, polyethylene for plastics, alcohol, mustard gas, and other organics. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 Under high pressure, and in the presence of a catalytic metal (platinum, rhodium, nickel), hydrogen reacts with ethylene to form ethane. Natural sources of ethylene include both natural gas and. Natural sources of ethylene include both natural gas and. Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type of hydrocarbon characterized by at least one carbon. 乙烯 (英語: ethylene)是由两个 碳 原子和四个 氢 原子组成的化合物。 两个碳原子之间用双键连接。 To manufacture ethylene oxide, polyethylene for plastics, alcohol, mustard gas, and other organics. This explains why some. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type of hydrocarbon characterized by at least one carbon. Ethylene is used in the production of fabricated plastics, antifreeze; 乙烯 (英語: ethylene)是由两个 碳 原子和四个 氢 原子组成的化合物。 两个碳原子之间用双键连接。 This explains why some fruits ripen faster if. This explains why some fruits ripen faster if. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. Ethylene is used primarily as an intermediate in. Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type. Ethylene (ethene), a gas of the formula ch 2 =ch 2, produced by fruit as a hormone to speed ripening of climacteric fruits. Natural sources of ethylene include both natural gas and. To manufacture ethylene oxide, polyethylene for plastics, alcohol, mustard gas, and other organics. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million. This explains why some fruits ripen faster if. Under high pressure, and in the presence of a catalytic metal (platinum, rhodium, nickel), hydrogen reacts with ethylene to form ethane. Ethylene is used primarily as an intermediate in. Ethylene (ethene), a gas of the formula ch 2 =ch 2, produced by fruit as a hormone to speed ripening of climacteric fruits.. Ethylene is used in the production of fabricated plastics, antifreeze; Natural sources of ethylene include both natural gas and. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 This explains why some fruits ripen faster if. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. Ethylene is used primarily as an intermediate in. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. To manufacture ethylene oxide, polyethylene for plastics, alcohol, mustard gas, and other organics. This explains why some fruits ripen. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016 [8]) exceeds that of any other organic compound. 最早报道的植物对乙烯的反应是“ 三重反应 ”(triple response of ethylene) [8],该反应的作用有:①抑制茎的伸长生长;②促进茎和根的增粗;③促进茎的横向增长。 乙烯 (英語: ethylene)是由两个 碳 原子和四个 氢 原子组成的化合物。 两个碳原子之间用双键连接。 Ethylene is used primarily as an intermediate in. Ethylene is a colorless gas with the chemical formula c₂h₄, making it the simplest alkene — a type of hydrocarbon characterized by at least one carbon. Ethylene is used in the production of fabricated plastics, antifreeze; Ethylene (ethene), a gas of the formula ch 2 =ch 2, produced by fruit as a hormone to speed ripening of climacteric fruits. To manufacture ethylene oxide, polyethylene for plastics, alcohol, mustard gas, and other organics.Ethylene Glycol Refractometer Chart at Annabelle Parkhill blog
Antifreeze Ethylene Glycol Percentage at Linda Marshall blog
Ethylene Glycol Refractometer Chart at Annabelle Parkhill blog
DSC melting and freezing points of aqueous ethylene glycol with the... Download Scientific Diagram
FreezePointChartGlycoChillEthyleneGlycolHeatTransferFluid Download Free PDF Density
Heat Transfer Fluids Dynalene, Inc.
Ethylene Glycol Chart
Boiling Point Of 50 50 Antifreeze
FREEZING POINT DATA FOR AQUEOUS SOLUTIONS OF ETHYLENE GLYCOL (MEG) Download Table
FREEZING POINT DATA FOR AQUEOUS SOLUTIONS OF ETHYLENE GLYCOL (MEG) Download Table
This Explains Why Some Fruits Ripen Faster If.
Under High Pressure, And In The Presence Of A Catalytic Metal (Platinum, Rhodium, Nickel), Hydrogen Reacts With Ethylene To Form Ethane.
Natural Sources Of Ethylene Include Both Natural Gas And.
Related Post: