Electronegativity Elements Chart
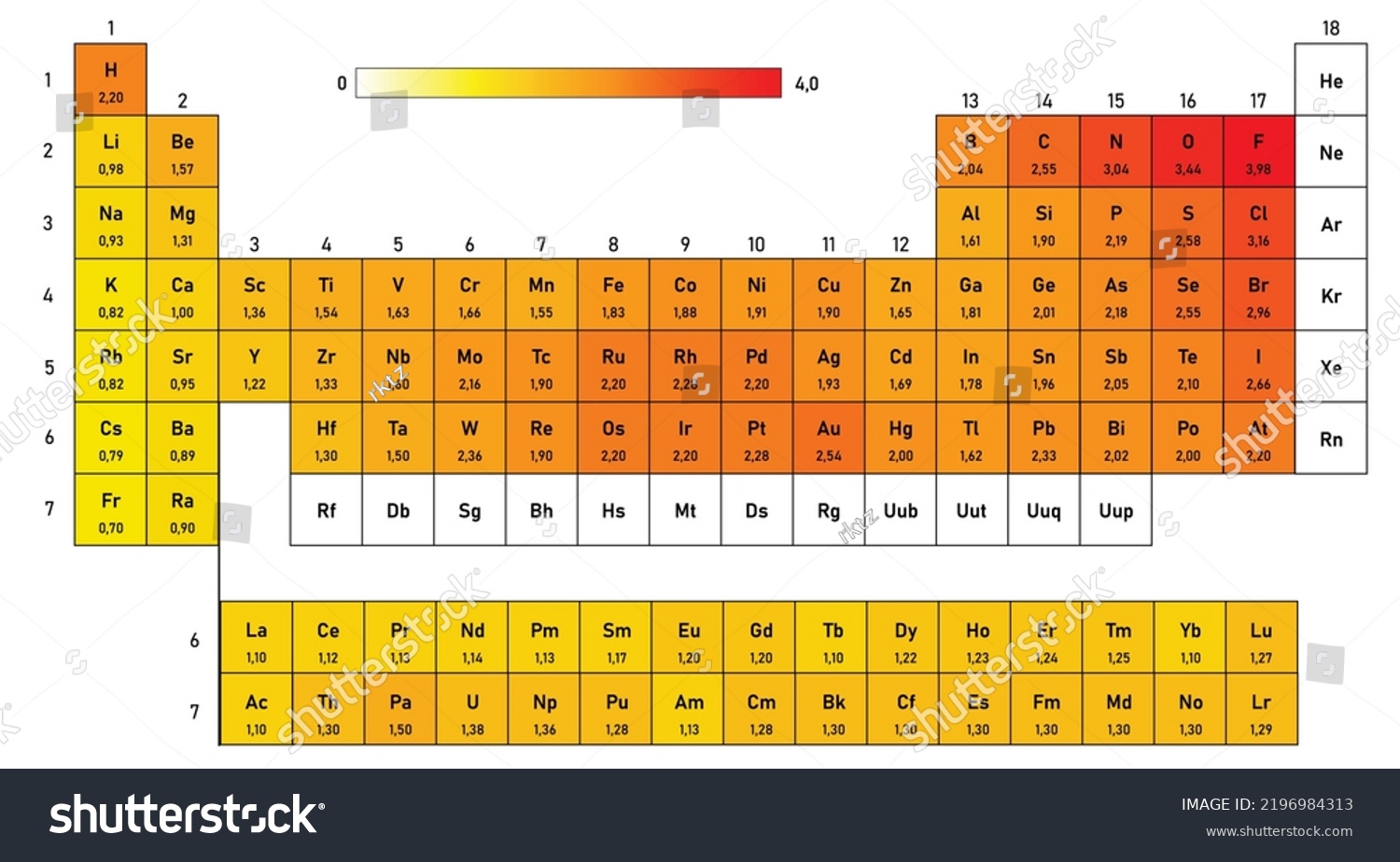
Electronegativity Elements Chart - Covalent bonds, and discover how to predict. I guess this electronegativity comes from a few aspects. Learn what ionic character is. The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic. However, the difference in electronegativity between. See an electronegativity example, and discover how to find electronegativity using the right tools for. Noble gases are supposed to be happy with the amount of electrons they have, because they have 8 valence electrons (thus, most. If we take the most simple definition, that it is the sum of the electron affinity and the ionization energy (divided by. Why do krypton and xenon have high electronegativity? Electronegativity increases across a period Trace ionic character trend on the periodic table, examine ionic vs. Covalent bonds, and discover how to predict. However, the difference in electronegativity between. I guess this electronegativity comes from a few aspects. The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic. In this sense, elements are less electronegative (or more electropositive) as you go down any group in the periodic table. Electronegativity increases across a period If we take the most simple definition, that it is the sum of the electron affinity and the ionization energy (divided by. See an electronegativity example, and discover how to find electronegativity using the right tools for. Why do krypton and xenon have high electronegativity? See an electronegativity example, and discover how to find electronegativity using the right tools for. In this sense, elements are less electronegative (or more electropositive) as you go down any group in the periodic table. Learn what ionic character is. 1 following is from wikipedia electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element. Electronegativity increases across a period Learn what ionic character is. I guess this electronegativity comes from a few aspects. Why do krypton and xenon have high electronegativity? Trace ionic character trend on the periodic table, examine ionic vs. However, the difference in electronegativity between. Trace ionic character trend on the periodic table, examine ionic vs. 1 following is from wikipedia electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a. The trend in electronegativity can be seen on the periodic table and, more. In the 1930s, scientist linus pauling proposed a scale to measure and explain the attraction atoms have for valence electrons in bonds. However, the difference in electronegativity between. The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic. See an electronegativity example, and. I guess this electronegativity comes from a few aspects. Learn what ionic character is. If we take the most simple definition, that it is the sum of the electron affinity and the ionization energy (divided by. Electronegativity increases across a period Trace ionic character trend on the periodic table, examine ionic vs. 1 following is from wikipedia electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a. Trace ionic character trend on the periodic table, examine ionic vs. Covalent bonds, and discover how to predict. Noble gases are supposed to be happy with the amount of electrons. However, the difference in electronegativity between. In the 1930s, scientist linus pauling proposed a scale to measure and explain the attraction atoms have for valence electrons in bonds. See an electronegativity example, and discover how to find electronegativity using the right tools for. 1 following is from wikipedia electronegativity, symbolized as χ, is the tendency for an atom of a. In the 1930s, scientist linus pauling proposed a scale to measure and explain the attraction atoms have for valence electrons in bonds. Trace ionic character trend on the periodic table, examine ionic vs. However, the difference in electronegativity between. Noble gases are supposed to be happy with the amount of electrons they have, because they have 8 valence electrons (thus,. In this sense, elements are less electronegative (or more electropositive) as you go down any group in the periodic table. Covalent bonds, and discover how to predict. The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic. Why do krypton and xenon have. In this sense, elements are less electronegative (or more electropositive) as you go down any group in the periodic table. I guess this electronegativity comes from a few aspects. However, the difference in electronegativity between. Noble gases are supposed to be happy with the amount of electrons they have, because they have 8 valence electrons (thus, most. 1 following is. 1 following is from wikipedia electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a. Why do krypton and xenon have high electronegativity? The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic. The trend in electronegativity can be seen on the periodic table and, more specifically, in the following graphs. In this sense, elements are less electronegative (or more electropositive) as you go down any group in the periodic table. Trace ionic character trend on the periodic table, examine ionic vs. Electronegativity increases across a period In the 1930s, scientist linus pauling proposed a scale to measure and explain the attraction atoms have for valence electrons in bonds. I guess this electronegativity comes from a few aspects. However, the difference in electronegativity between. Learn what ionic character is. Covalent bonds, and discover how to predict.Electronegativity Table Elements Color Code Stock Vector (Royalty Free) 2196984313 Shutterstock
Electronegativity Chart Printable periodic table of the elements
Electronegativity Chart Printable periodic table of the elements
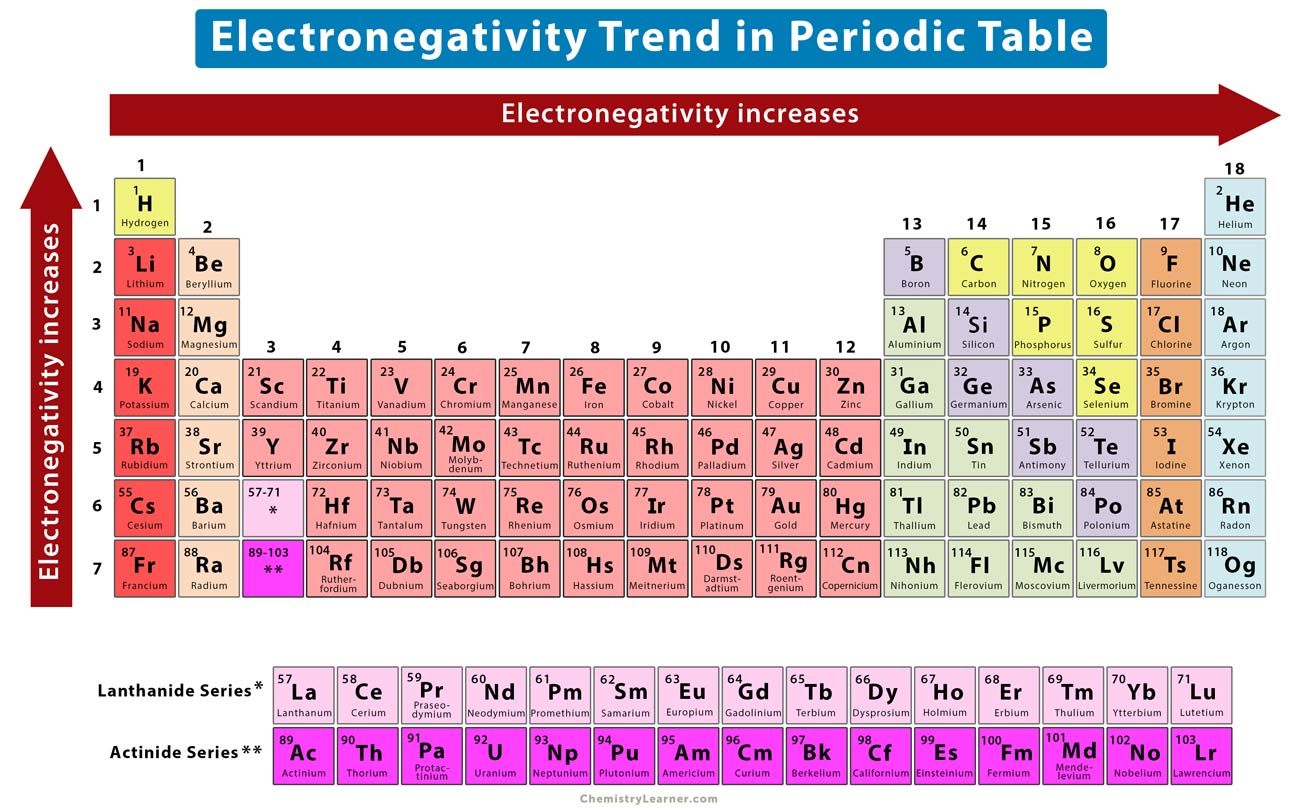
Electronegativity Definition, Value Chart, and Trend in Periodic Table
Electronegativity Definition and Trend Periodic table, Important life lessons, Periodic table
Electronegativity Chart Printable periodic table of the elements
Electronegativity and Electronegativity Chart in PDF
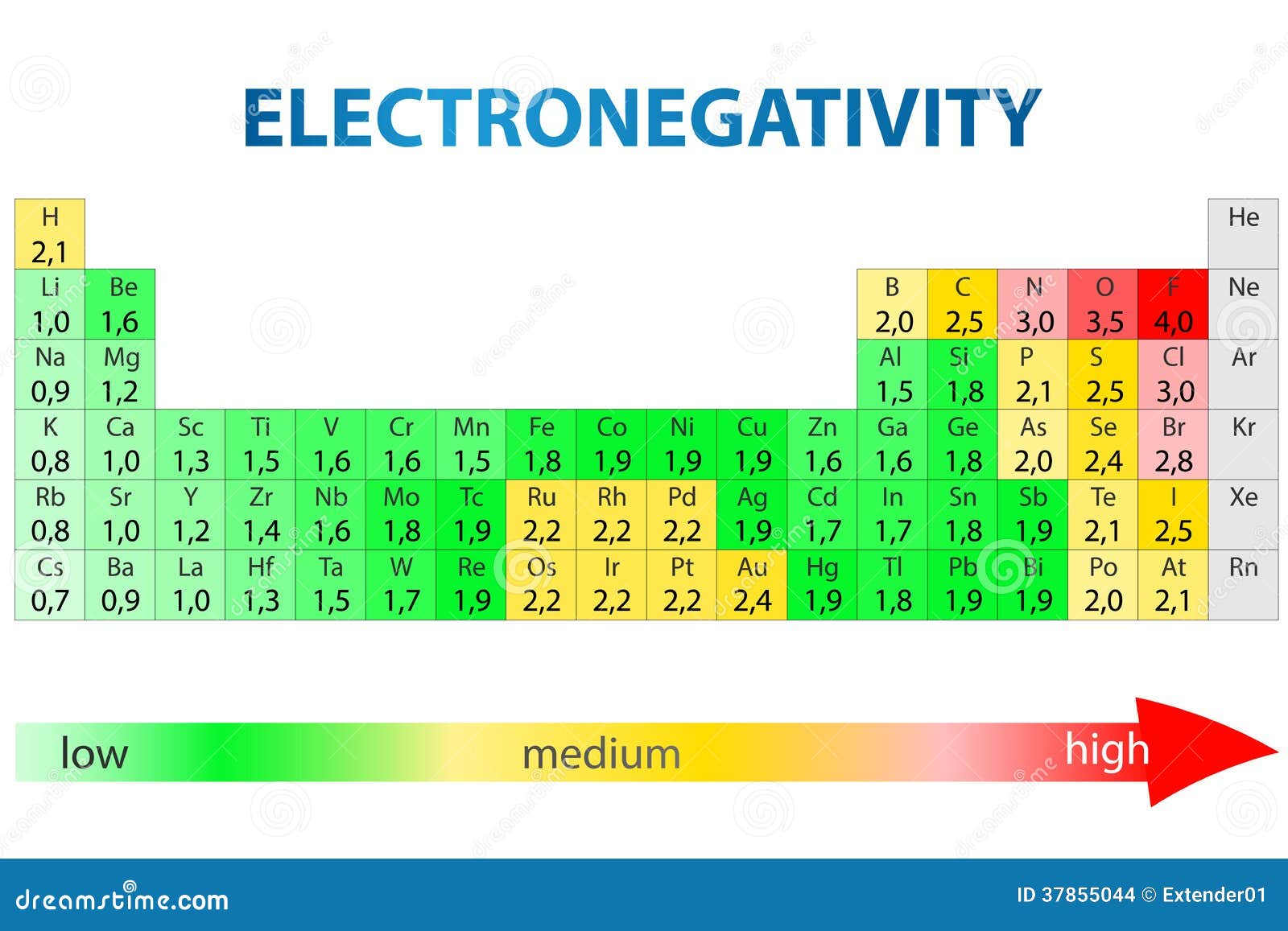
Electronegativity Periodic Table Stock Photo Image of elements, electrons 37855044
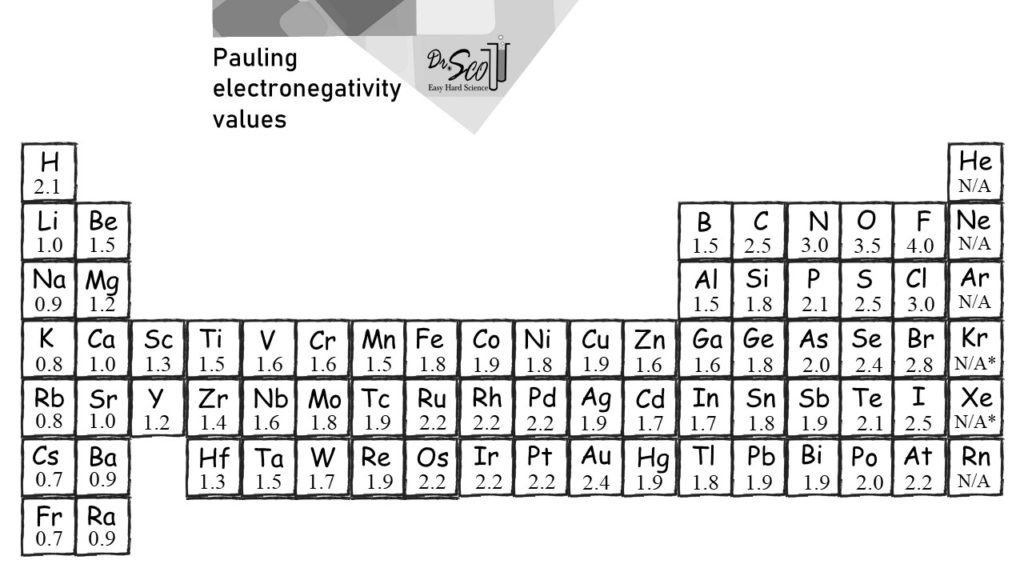
Periodic Table of Electronegativities
Printable Periodic Table of the Elements Electronegativity
If We Take The Most Simple Definition, That It Is The Sum Of The Electron Affinity And The Ionization Energy (Divided By.
Noble Gases Are Supposed To Be Happy With The Amount Of Electrons They Have, Because They Have 8 Valence Electrons (Thus, Most.
See An Electronegativity Example, And Discover How To Find Electronegativity Using The Right Tools For.
Related Post:




.PNG)
/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png)